Atlas page 29
The Acid Fast Stain was developed for the GENUS MYCOBACTERIUM which contain species that cause Tuberculosis, Leprosy, and other fatal illnesses. We use one of two non-pathogenic Mycobacterium in our classes for demonstration.
Mycobacterium are notoriously hard to kill and hard to stain; they also grow very very slowly. All of these characteristics of the microbe are due to the veryTHICK LIPID LAYER in the cell wall. As you might expect, the Mycobacterium stain Gram-positive because of the THICK cell wall. REMEMBER... the peptidogycan layer in Mycobacteria is THIN but it stains Gram variable on the positive side (almost all Gm+).
Smear from Agar comes 1st - the STEPS:
ALL STAINS START WITH THE PROPER PREPARATION OF A SMEAR (from agar usually):
1. Disinfect your table-top and obtain your incomplete Universal Precautions Lab Clothing. Clean a slide WELL, dry the cleaned slide. Using your Sharpee, place a dime-sized circle in the center on the BOTTOM SIDE of the slide...
2. Heat your loop to red hot and cool it...
3. "Capture" a drop of water on your loop...
4. Spread the water on top of the circled area of the slide...
5. Heat your loop to red hot and cool it...
6. Obtain a culture on a test tube agar slant (or from a colony on an agar plate - for other preps see "Preparation of a Smear from a Broth")...
7. Flame the test tube lid gently, remove the lid with your "pinky-finger" of the hand holding your loop and hold the lid in your hand (NEVER lay a top or loop on a table top!). Flame the LIP of the test tube gently. Insert your loop into the test tube and go PAST the slant where the microbe is growing on the agar as a "film." Lower the loop and gently pull it toward you as it glides gently across the SURFACE of the slant picking-up a small amount of gelatinous microbe film inside the loop. DO NOT GET THE AGAR GEL ON YOUR LOOP!
8. Flame the lip of the test tube again as well as the lid; then, replace the test tube lid and place it in a test tube rack.
9. SMEAR your loop material into the water on the circled area of your slide. MIX the water and microbe until the liquid is SLIGHTLY CLOUDY.... SPREAD that cloudy liquid outside the circle as much as possible to aid in AIR DRYING!
10. Heat and cool your loop and place it in a test tube rack - DO NOT LAY IT DOWN ON THE TABLE-TOP!
11. AIR DRY THE CLOUDY LIQUID ON THE SLIDE COMPLETELY!
12. HEAT FIX the slide by passing it through a flame until the slide is almost too hot to your naked skin... If you over heat fix the microbes will EXPLODE. If you under hear fix the microbes will be washed down the drain during staining!
CURRENT (ALTERNATE) ACID FAST STAINING STEPS:
1. Obtain a properly made SMEAR with the small circle draw 3/4th of the way down the slide on the bottom of the slide (this allows you place to HOLD the slide when passing the primary stain through the flame).
2. FLOOD the circle with the PRIMARY stain - make a "bubble" of primary stain (Acid Fast = Carbolfuchsin)
3. Pass the slide through the Bunsen burner flame until your slide gets WARM and the bubble of stain "withdraws" slightly - evaporates some - about 5 X - NOTE: You should put a 1 ft square of paper towel under your Bunsen Burner to collect any spilled stain)
4. Acid Fast shake off the primary stain gently, obtain 1/4 to 1/2 dropper full of Acid Alcohol and spray it above the circle; WASH immediately with Water by aiming the stream ABOVE the smear... Do NOT Overwash!
5. Place the slide on the staining rack and COUNTER STAIN with Methylene Blue for 2-3 min. Rinse lightly with water, BLOT dry and add immersion oil.
=======================================================================
INTERPRETATION CAUTIONS:
CAUTION: ANY PRIMARY STAIN THAT IS "heated" will stick to "HARD TO STAIN" cell parts... ENDOSPORES & the lipids of MYCOBACTERIA!
CAUTION: 1- ONE HOT PINK ROD makes the stain Acid Fast (otherwise known as Acid Fast Positive). This is because Mycobacteria are hard to stain... you will see clumps of rods... and only a few are fuchsia or hot pink.
CAUTION: sometimes the ENDOSPORES will take-up the primary stain and appear like HOT PINK ovals.. this is still an acid fast negative or non-acid fast stain because we are staining CELLS.... endospores are NOT CELLS and thus do not count.
CAUTION: if everything appears Baby-blue, the the stain is acid fast negative or non-acid fast.
RESULTS:
The Acid Fast Stain utilizes Carbolfuchsin (primary stain) which is a red-purple stain that has an affinity for lipids. The counterstain is Methylene blue. AF+ microbes vegetative cells stain as fuchsia colored sharp-ended rods while AF- microbes cells stain baby blue.
===============================================
USE THE ALTERNATE PROCEDURE FOR THE ACID FAST STAIN
PROCEDURE:
1. Make a typical smear.
2. After heat-fixing, cover the circled smear with Carbolfuchsin stain. DO NOT PUT TOO MUCH STAIN.
3. Hold the slide over the bunsen burner until the the slide gets HOT AND about 20% of the primary stain has evaporated (NOT ALL OF IT!!!!!). Constantly move the slide in and out of the flame or up and down. Put paper towel under the Bunsen burner to catch any "spills." SHAKE off the excess PRIMARY STAIN after heating... then...
5. Put 1/2 dropper full of ACID ALCOHOL on the smear and immediately wash it off with water...DO NOT OVERWASH!
6. Counter stain with METHYLENE BLUE for 3-4 min. or so. Wash with water... Blot dry, add immersion oil and focus under 10X
7. View under oil immersion – 100X, draw.|
|
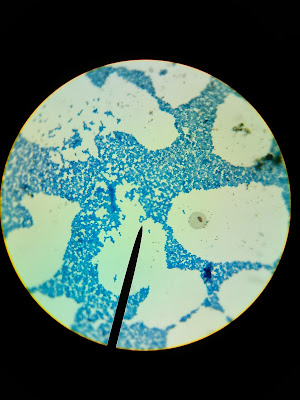
PHOTO of the sharp- ended Mycobacterium rods which are ACID FAST POSITIVE (bright fuchsia colored) on an ACID FAST STAIN - NOTE: that not ALL the microbes will take the stain because of the impermeable lipid layers of the cell wall
To be AF+ you must have 1 baby-blue rod and at least 1 hot pink rod ALL hot pink is over cooked |
| Acid Fast rods... of M. leprai |
COMMON ERRORS IN Acid Fast Staining:
A microbe is considered Acid Fast Positive when ANY sharp-ended rods are Fuchsia in color because this microbe is very difficult to stain - you must SCAN the whole slide to decide.
Another common error is calling an endospore a cell and erroneously deciding that the microbial culture is Acid Fast Positive. All difficult to stain "things" can take-up the carbolfuchsin and endospores may stain fuchsia! Mycobacteria are rods!
In the Acid Fast Staining procedure, the decolorizer is ACID ALCOHOL not acetone or ethanol as it is in other stains.
Don't boil off all the PRIMARY STAIN.. only about 20-25%!
Don't let put too little PRIMARY STAIN ON THE SMEAR!
Don't over-wash or under-wash!
|